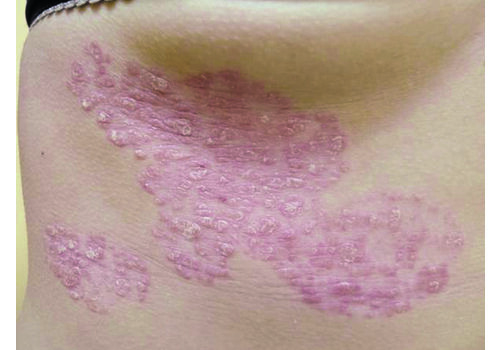
Psoriasis Treatment (RecoSMA® on the MULTILINE™ Platform)

LINLINE manages stable plaque psoriasis with RecoSMA®, a non-thermal Er:YAG method designed to clear plaques, calm inflammation, and improve quality of life—while keeping downtime short. In selected cases, we also apply a KTP 540 nm program to help normalize microcirculation and reduce residual erythema.
Why this works
- Non-thermal, dual action: RecoSMA® uses an Er:YAG 2.94 µm emitter with a Spatially Modulated Ablation (SMA) head that creates microscopic epidermal micro-ablations and launches acoustic waves. These waves enter the dermis, producing mechanical micro-stimulation (not heat) that disrupts pathological structure and supports reverse resolution of plaques.
- Dermal reach without burning: Micro-events at the surface drive wave energy into the dermis (up to ~6 mm), separating abnormal fibers and stimulating regeneration—all without thermal damage to basal layers. Histology shows hyalinization/gelification patterns and interfibrillar separation rather than coagulative burns.
- Microcirculation support: When needed, MULTILINE™’s KTP 540 nm program coagulates dilated capillaries, helping normalize local flow and reduce inflammatory drive in the plaque environment.
Who is it for?
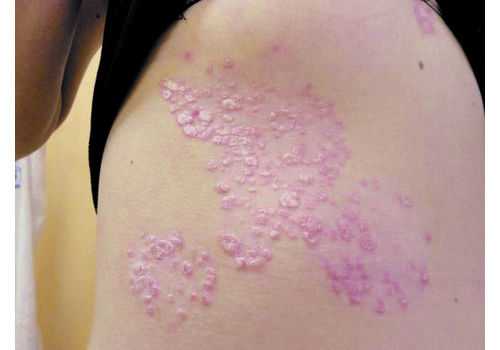
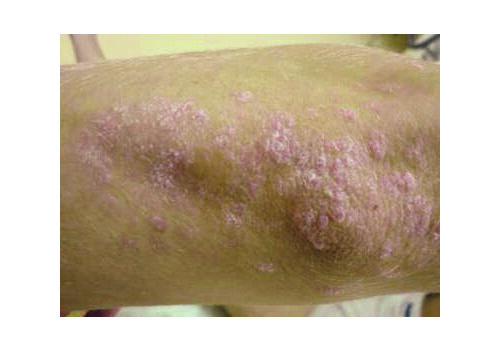
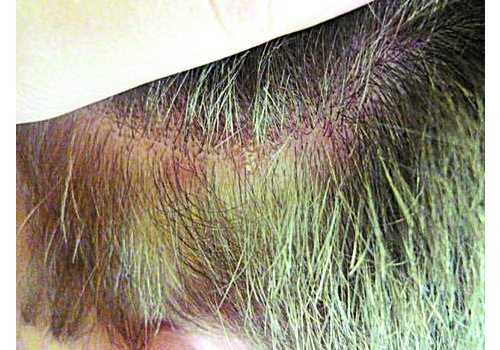
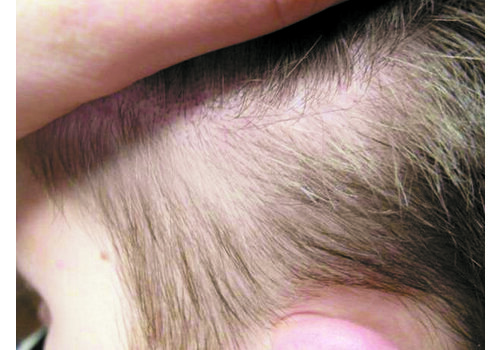
- Indications: Confirmed psoriasis vulgaris (localized or more widespread), seborrheic or skin-fold psoriasis, including scalp; stationary or regressive stage; typically mild–moderate severity.
- Not for: Progressive/unstable disease, guttate, pustular, or erythrodermic psoriasis; PASI >20; significant decompensated comorbidities. (Your clinician will screen.)
Evidence & outcomes
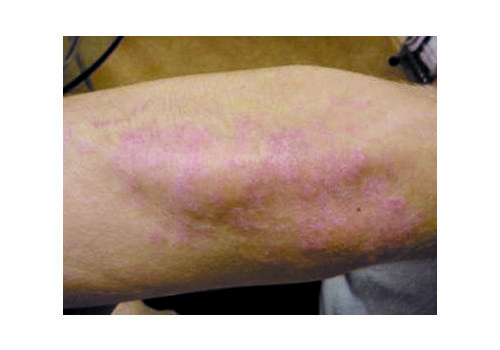
- In a prospective case series (n=112), RecoSMA® achieved clinical remission in 87.5%, with significant PASI and DLQI improvements; mean PASI dropped 86.9% (8.4 → 1.1; p=.002) and DLQI improved 72% (7.9 → 2.21; p=.004). Some patients reported remissions lasting up to 4 years at follow-up. Average 2.7 sessions (range 1–7). No complications were observed.
- Histology across biopsied plaques documented stepwise normalization from week 1 to post-course (reduced infiltration, restoration of granular layer, resolution of edema).
What treatment looks like
- Expect brief warmth/tingling; post-procedure a white dense frost appears, then a thin crust (day 1). Peeling peaks days 5–7, subsiding within 1–3 days. Most daily activities continue; standard gentle skincare applies.
- No burns/scars expected with protocol (non-thermal mode); dyspigmentation, when seen, relates to inflammation rather than melanin injury.
Benefits at a glance
For patients
- High clearance rates with few sessions; short social downtime.
- Comfortable, non-thermal approach—no burning of healthy skin.
- Quality-of-life gains (DLQI) and long remissions reported in follow-up.
For practitioners
- Reproducible endpoints (PASI/DLQI) and clear histologic rationale.
- Simple workflow on MULTILINE™; add KTP 540 nm when capillary burden persists.
- Low complication profile when protocols are followed.
Key takeaway
For stable plaque psoriasis, RecoSMA® provides non-thermal plaque clearance and dermal normalization with minimal downtime and strong patient-reported benefits—on a platform that also lets clinicians address microvascular load where needed with KTP 540 nm.





 06.10.2025
06.10.2025